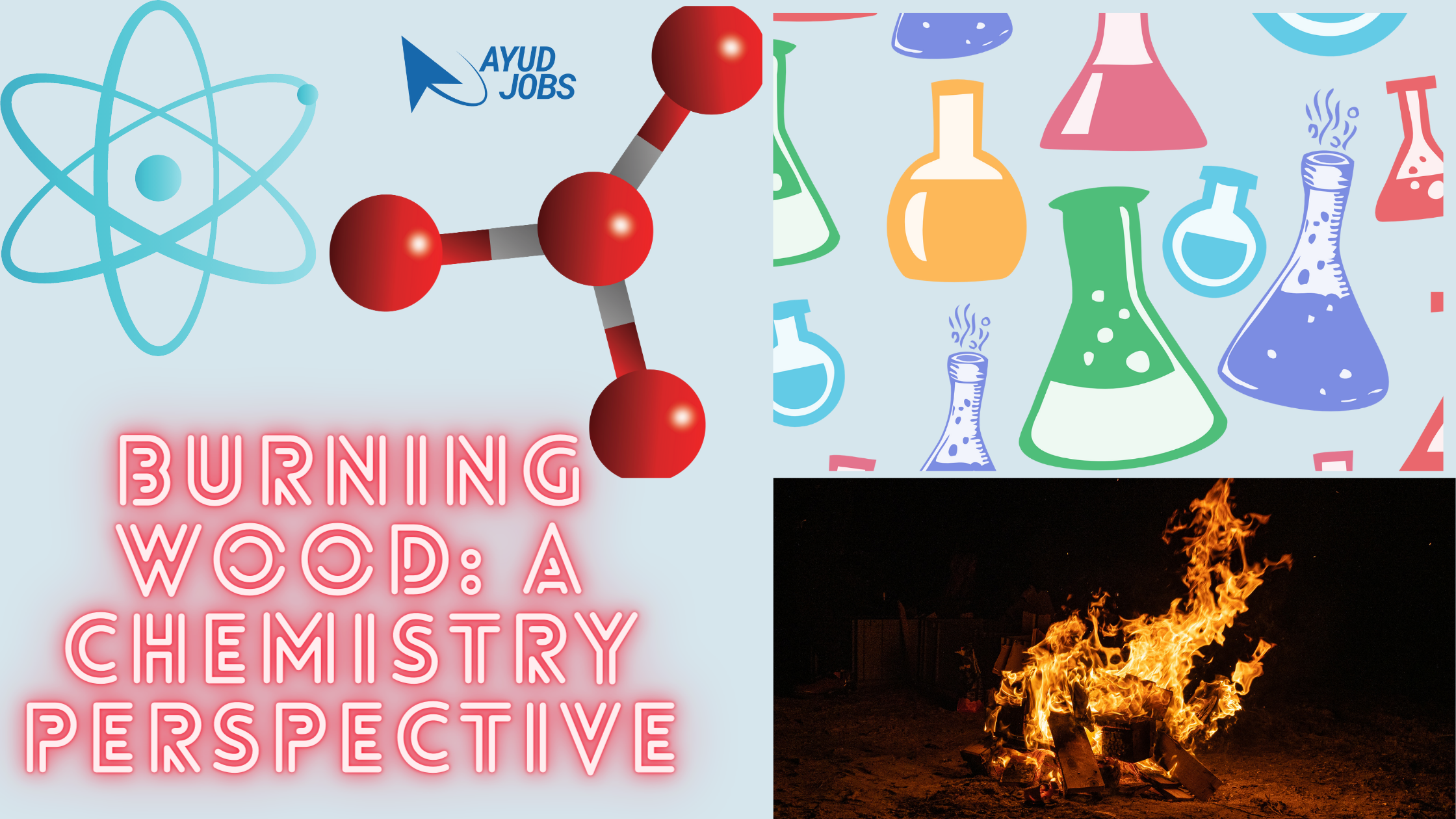
Burning Wood: A Chemistry Perspective
Burning Wood: A Chemistry Perspective
Introduction:
Burning wood is an age-old practice that evokes a sense of warmth and comfort. But have you ever wondered about the captivating chemistry that lies within this familiar sight? In this blog, we will embark on an enlightening journey into the world of burning wood, exploring its chemistry from a unique perspective. Whether you’re a science enthusiast or simply curious about the scientific wonders of everyday life, join us as we unravel the secrets behind the flames.
Understanding Combustion:
The Chemistry Unleashed At its core. Combustion is the chemical process that burns wood. It occurs when wood, acting as a fuel, reacts with oxygen in the air. Let’s delve into the captivating chemistry that drives this phenomenon and gain a comprehensive understanding of its intricacies.
Decoding the Chemical Equation:
Let’s summarize the chemical equation that represents the combustion of wood as follows:
Wood + Oxygen → Carbon Dioxide + Water + Heat
Now, let’s break down each component of this equation to grasp the underlying chemistry that unfolds during the burning of wood.
Wood:
Unveiling the Complex Composition. Wood primarily consists of cellulose and lignin, making it a complex organic material. These compounds serve as the fuel for combustion, providing carbon, hydrogen, and oxygen atoms necessary for the reaction.
Oxygen:
The Energetic Partner in Combustion Oxygen. An abundant gas in the air we breathe, acts as the crucial partner in combustion. It serves as an oxidizing agent, reacting with the carbon and hydrogen in wood to initiate the chemical reaction.
Carbon Dioxide:
From Wood to the Atmosphere During combustion, carbon from the wood combines with oxygen to form carbon dioxide (CO2). Excessive emissions of carbon dioxide, an odorless and colorless gas, are released into the atmosphere, contributing to climate change. Understanding this process is vital in developing cleaner energy alternatives.
Water:
The Unexpected Product As the hydrogen atoms in wood react with oxygen, they form water molecules (H2O). The release of water vapor adds to the visual spectacle of a crackling fire, with wisps of steam rising from burning logs.
Heat:
The Radiant Energy Combustion is an exothermic reaction, meaning it releases heat energy. The breaking and formation of chemical bonds within the wood result in the warmth and radiant energy associated with burning wood. We can harness this valuable heat for various applications, including heating homes and cooking food.
Implications and Beyond:
Unleashing the Potential, The chemistry behind burning wood has significant implications and broader significance:
Energy Production:
People have long utilized the burning of wood as a valuable source of heat and energy. Fireplaces, wood stoves, and biomass power plants harness the energy released during wood combustion to generate heat or electricity.
Environmental Considerations:
Understanding the chemistry of combustion enables scientists to develop cleaner and more sustainable energy sources. It also aids in comprehending the environmental impact of carbon dioxide emissions, fostering the search for eco-friendly alternatives.
Fire Safety:
Knowledge of combustion is instrumental in fire safety measures. Understanding the conditions that initiate and sustain fires allows for effective prevention and mitigation strategies, ensuring the safety of individuals and property.
Conclusion:
Burning wood is not merely a visual spectacle but a fascinating chemical process governed by the principles of chemistry. By delving into the science behind the flames, we gain a deeper appreciation for the energy transformations and environmental consequences associated with burning wood. Moreover, this knowledge empowers us to explore cleaner energy solutions and implement effective fire safety measures. Burning Wood: A Chemistry Perspective. So, the next time you find yourself captivated by a crackling fire, take a moment to marvel at the hidden chemistry unfolding before your eyes.
Check your knowledge on Chemistry by taking Mock Test.
MockTest – Explore / Burning Wood A Chemistry Perspective
https://ayud.page.link/RHBX
MockTest – Explore / Burning Wood A Chemistry Perspective 2
https://ayud.page.link/NsMr
Harnessing the Power of Discipline: A Pathway to Career Success.

What is Ayud Jobs?
Ayud Jobs is a one-stop platform for career development. It offers a wide array of services, including creating a career roadmap, building a resume, receiving matching job alerts, and staying updated on job fairs/drives and job openings. Their Blog section provides career-related articles, including job searching and interview strategies.
By registering with Ayud Jobs, you can access all these resources and stay up-to-date with the latest career trends. Their Event feature keeps you informed about education-related events, college and university events, health tips, and more. Furthermore, they provide practice multiple-choice questions (MCQs) to aid in interview preparation.
To take advantage of their comprehensive resources and stay informed about the latest career advice and opportunities, register with Ayud Jobs today.
Click here to install Ayud Jobs from Play Store.
#ayudjobs #ayudsoftware #BurningWood #CombustionChemistry #ChemicalEquation #WoodBurning #OxygenReaction #CarbonDioxide #WaterVapor #HeatEnergy #EnergyProduction #EnvironmentalImpact #FireSafety